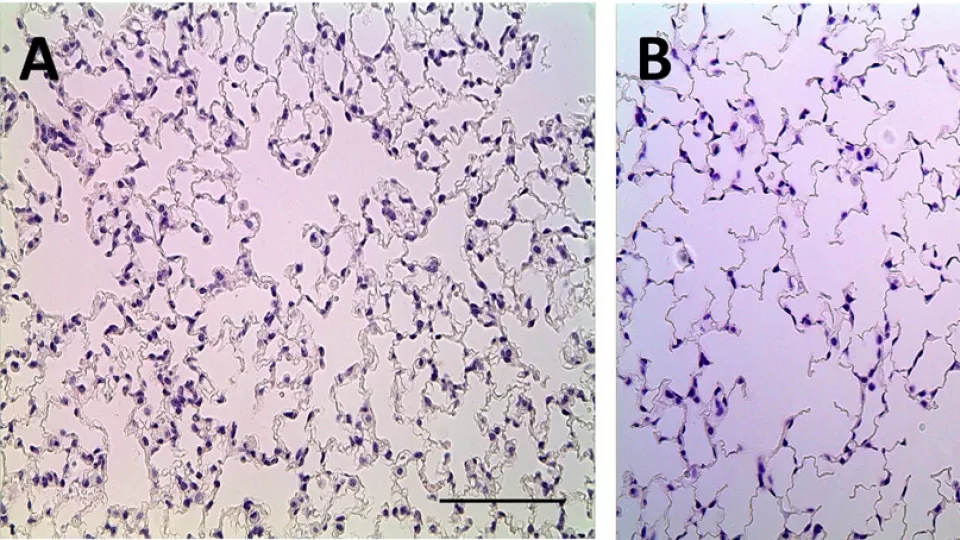
Normal lung (image A) compared to lung developed with low levels of VEGF (image B) demonstrating abnormal air spaces and lung tissue. Photo courtesy of Grikscheit Lab, The Saban Research Institute of Children’s Hospital Los Angeles
Baby’s Breath: A New Way to Study Neonatal Lung Disease
Normal lung (image A) compared to lung developed with low levels of VEGF (image B) demonstrating abnormal air spaces and lung tissue. Photo courtesy of Grikscheit Lab, The Saban Research Institute of Children’s Hospital Los Angeles
Investigators at The Saban Research Institute of Children’s Hospital Los Angeles have created a novel model for studying a lung disorder of newborn babies. Their study, published in PLOS ONE on February 10, describes the first model that allows investigators to consider the chronic effects of developmental lung disease.
Neonatal respiratory distress syndrome (RDS) is a restrictive lung disease characterized by insufficient surfactant and lung immaturity. Surfactant plays multiple important roles in the lung, such as facilitating lung expansion, preventing air spaces collapse, and helping fight infection. Babies born with RDS are often given supplemental surfactant and require a ventilator to help them breathe. Additionally, these babies often have other medical issues such as prematurity.
Previous studies have reported that babies with RDS, and adults with other lung diseases, have low levels of vascular endothelial growth factor (VEGF), a signalling protein that promotes the growth of new blood vessels. It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate. In the lung mesenchyme, it is known to have a central role in formation of the lung’s branching structure as well as alveoli and blood vessel development.
To develop an animal model that studies exactly how VEGF affects lung development, it has been necessary to duplicate the comorbidities seen in children, like premature delivery and mechanical ventilation. However, these conditions result in a model that has too many variables, making it difficult to study the effects of abnormal VEGF. Additionally, many studies have evaluated changes only in the first few weeks of life, which does not provide information about long-term changes to the lung and overall health trajectory of the individual.
The model developed at CHLA studied full-term mice with a genetic manipulation to evaluate the long-term effects of abnormal VEGF levels. The investigators were able to observe the mice for three months, until lung development was complete.
“By ‘turning down’ VEGF signaling, we found that it was enough to ‘turn on’ lung disease, even in full term animals who weren’t on oxygen and didn’t have other problems,” said Minna Wieck, MD, an investigator and surgical resident at CHLA and first author on the study. By overproducing – known as “overexpressing” – a decoy receptor that binds VEGF, the team at CHLA was able to replicate the key features of RDS, including low surfactant levels. Ultimately, when mice with this genetic change grew into adults, they demonstrated abnormal restrictive lung function. This may have important implications in the treatment and prognosis of children with neonatal lung disease.
“Babies with respiratory problems often grow up to be adults with respiratory problems,” said Tracy Grikscheit, MD, a pediatric surgeon and principal investigator at The Saban Research Institute of CHLA. “Our goal is to be able to find new ways to intervene very early to significantly impact the quality of life for our patients. Now we can investigate the various factors that severely impact the lung development of premature babies and to more specifically target future human therapies. his model mimics the condition and will allow scientists to better determine the mechanism that drives long-term effects that can lead to disabling disease.” Grikscheit is senior author on the study.


