Could a Key Protein Lead to New Therapies for Wilms Tumor?
By most accounts, Wilms tumor treatment is a success story. For those younger than 20 who develop this most common form of childhood kidney cancer, five-year survival rates are over 90%.
But for the roughly 15% of children with Wilms tumor who relapse—or for those whose cancer does not respond to treatment—survival rates plummet. These are the patients that Astgik Petrosyan, PhD, aims to help.

Dr. Petrosyan—an investigator in the GOFARR Laboratory for Organ Regenerative Research and Cell Therapeutics in Urology at Children’s Hospital Los Angeles—recently received the 2024 Societies for Pediatric Urology Research Grant. The award supports her studies into a critical, but often overlooked, area of this cancer: the tumor microenvironment.
“We’re not just looking at the cancer cells themselves,” she explains. “We’re looking at the environment around those cells—the extracellular matrix—which might be helping the tumor grow and resist treatment.”
Blocking a key protein
Dr. Petrosyan and her team have identified a specific protein in the extracellular matrix called COL2A1. This protein, she believes, could be a key reason why some Wilms tumors are so hard to treat.
The team’s early findings suggest that COL2A1 helps tumor cells act like cancer stem cells. Cancer stem cells are particularly dangerous because they can keep dividing, resist chemotherapy, and cause the cancer to return.
“COL2A1 appears to strengthen the environment around the tumor cells, allowing them to possibly grow more aggressively and making it harder for treatments to work,” she says.
Dr. Petrosyan’s team is investigating COL2A1’s role in Wilms tumor by blocking the protein in patient-derived lab-grown tumor cells to see if it halts growth or improves chemotherapy sensitivity. They also do drug testing in mice with human Wilms tumors to determine if reducing COL2A1 levels slows tumor growth.
“By blocking COL2A1—either in the lab or in mouse models with human tumors—I expect to see tumors shrink, become more sensitive to chemotherapy, and lose their ability to grow and spread,” she says.
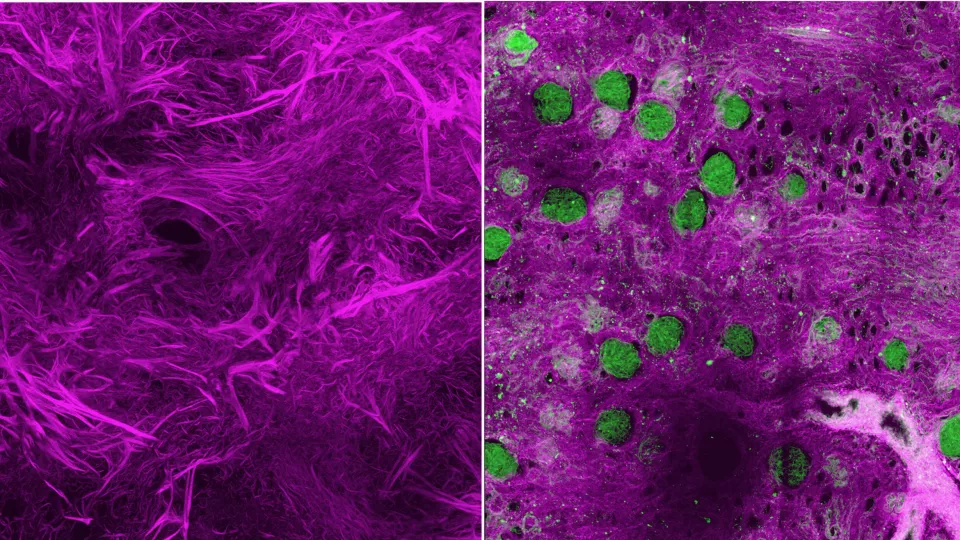
Left: A decellularized human Wilms tumor sample, imaged to show a dense web of disorganized fibrillar collagens (in magenta). Right: A decellularized normal human kidney specimen with fibrillar collagens imaged in magenta and glomeruli highlighted in green.
Paving the way for targeted therapies
She adds that this research benefits from the GOFARR Lab’s close collaboration with clinicians and surgeons in the Division of Urology at CHLA.
“I’m a basic scientist, but it is so valuable to have the perspective of our clinicians who are treating children with Wilms tumor,” Dr. Petrosyan notes. “We also use patient tumor samples in our research, which helps us study this cancer in a much more realistic way.”
Ultimately, her goal is to pave the way for more targeted therapies for children with Wilms tumor—and possibly other cancers.
“This protein has also been found in other cancers, such as melanoma and certain types of prostate cancer,” she explains. “If we can find a way to block it, the potential for new therapies could extend beyond Wilms tumor.”


