Theranostics: Combining Cancer Therapeutics and Diagnostics
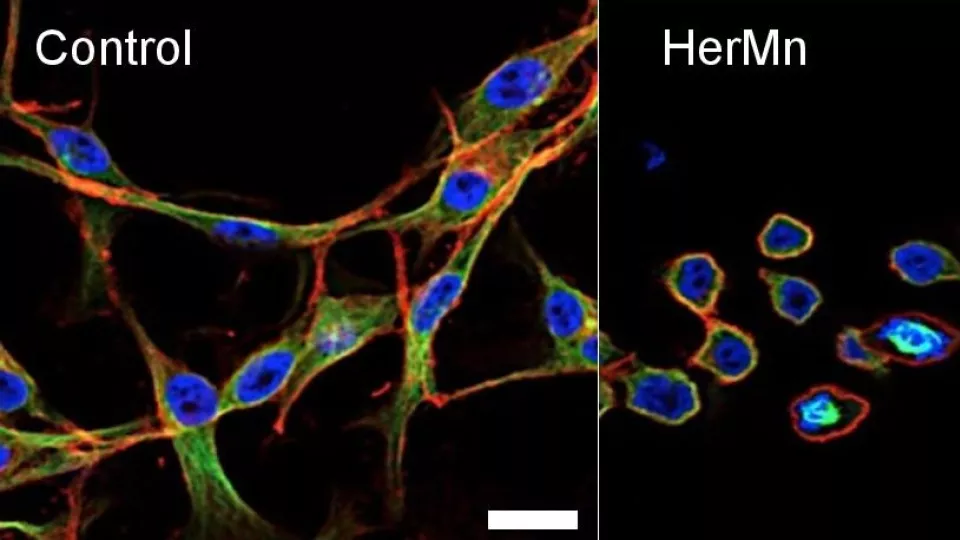
Left: Untreated cancer cells. Right: Cells treated with the nanoparticle complex HerMn, which is cytotoxic to tumor cells (disrupting the cytoskeleton and structure of these cells as shown above) while simultaneously enhancing MRI contrast. Image courtesy of Karn Sorasaenee, PhD, The Saban Research Institute, Children’s Hospital Los Angeles.
Researchers at The Saban Research Institute of CHLA, in collaboration with Cedars-Sinai Medical Center and the California Institute of Technology (Caltech), have unveiled a novel nanoparticle complex that induces tumor cell death while simultaneously enhancing contrast in MRI images of the tumors. The study, recently published in the Journal of Controlled Release, is one of several advancements in the emerging field known as theranostics, which seeks to combine therapeutics and diagnostics to target various diseases including cancer.
“This study is at the forefront of molecular imaging, probe development, and cancer targeted therapy,” says Karn Sorasaenee, PhD, an author on the paper and member of the Translational Biomedical Imaging Laboratory at The Saban Research Institute.
Theranostics involves chemically linking different molecules onto a scaffold, forming a complex. Each molecule can have different functions, from diagnostic or imaging to drug delivery and cell targeting. However, having too many molecules may result in some unexpected molecular function interference among one another.
To address this cross-interference, researchers used a corrole – a macrocyclic organic compound – bound to the metal manganese. Researchers targeted the corroles to tumor cells by attaching a recombinant protein that binds the human epidermal growth factor receptor protein (HER), which is often overexpressed on tumor cells. The protein-corrole complex formed the nanoparticle HerMn exhibiting toxicity against tumor cells. It disrupted the membranes of mitochondria, the powerhouses of cells, and damaged the cytoskeleton, the structural support of cells.
HerMn also enhanced contrast of MRI images as demonstrated by extensive MRI studies conducted at CHLA. MRI images are typically grayscale, and contrast-enhancing agents like HerMn improve the clarity of these images, allowing physicians and technicians to better identify and distinguish between various features.
More studies are needed to determine what side effects may result if any before the nanoparticle is available for clinical uses, according to Sorasaenee, but being able to use one compound to help simultaneously diagnose and treat tumors could prove incredibly beneficial.
“You can do imaging while simultaneously administering therapy, which can help you understand the treatment better,” says Sorasaenee.
This work was supported by grants from the National Cancer Institute and by funding from the National Center for Research Resources, now at the National Center for Advancing Translational Sciences.


