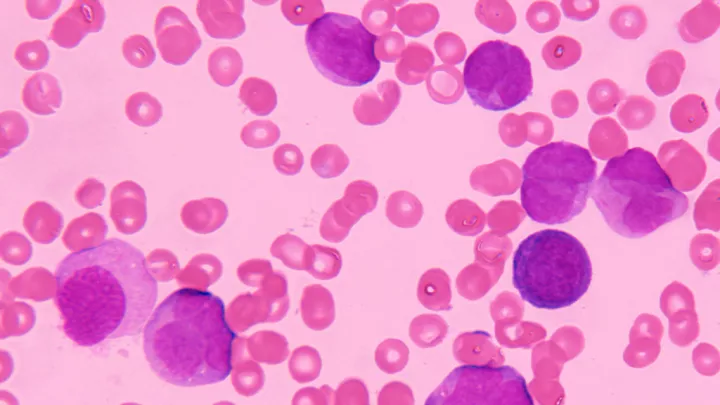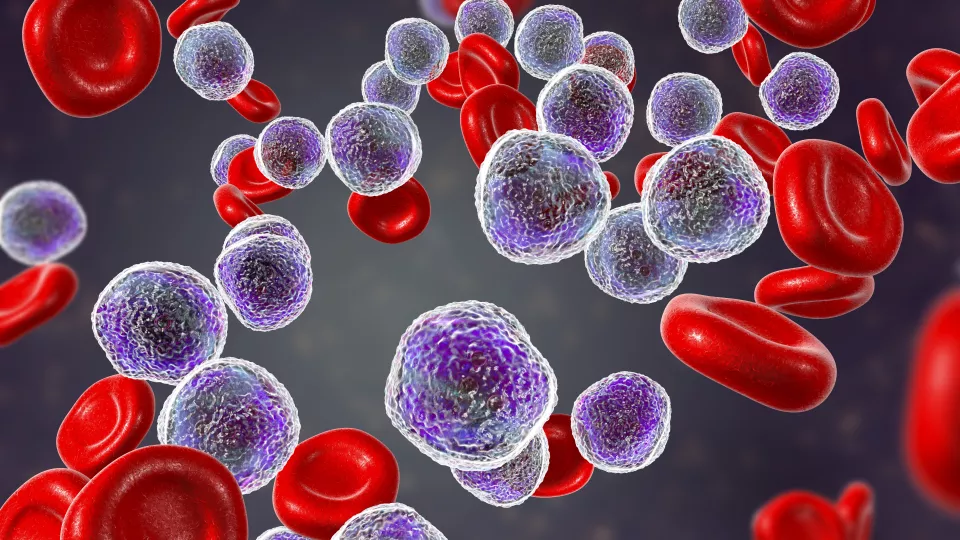
Acute lymphoblastic leukemia in bone marrow
Can a Novel Approach Lead to Less-Toxic Treatments for Leukemia?
Traditional cancer treatments such as chemotherapy and radiation can come with collateral damage. In the process of killing cancer, the treatments harm normal cells, too, leading to both short- and long-term side effects. Even today’s targeted drugs and immunotherapy can have effects on normal tissues.
But what if a therapy could be so specific, it only kills tumor cells? That’s the Holy Grail of cancer research—and a team from Children’s Hospital Los Angeles and USC recently became a step closer to reaching it.

The team—led by Michael Lieber, MD, PhD, of USC, who is collaborating with Yong-Mi Kim, MD, PhD, MPH, and Deepa Bhojwani, MD, of Children’s Hospital Los Angeles—is investigating whether inhibiting an enzyme called Artemis can cause leukemia cells to suffer fatal chromosome breaks, while sparing the body’s normal immune cells.
In their latest paper, published in Frontiers in Cell and Developmental Biology, the researchers showed that blocking Artemis significantly decreased the proliferation of B-acute lymphoblastic leukemia (ALL) tumor cells, while mature lymphocytes were not affected. The in vitro studies were performed on patient-derived B-ALL cells from CHLA.
“This would be a completely different way to kill cancer,” says Dr. Kim, an investigator in the Cancer and Blood Disease Institute at CHLA and co-senior author of the study with Dr. Lieber. “It’s a very fundamental biological approach. If it works, you may not even need chemotherapy.”
‘Dangerous acrobatics’
Dr. Lieber is spearheading the research—which has been funded through the prestigious National Cancer Institute Experimental Therapeutics (NExT) program for 10 years, and through the National Cancer Institute for 20 years. His idea: take advantage of “dangerous acrobatics” that leukemia cells continually undergo.
He explains that when normal lymphocytes—B cells and T cells—are developing, they go through a complex gene rearrangement process known as V(D)J recombination. In the final step of this process, two pieces of DNA must join together.
But each of these pieces has a special rounded closed end, called a “hairpin.” This DNA hairpin must first be opened for the two strands to join. Once the pieces merge, the lymphocyte is mature; it never undergoes this process again.
In contrast, leukemia cells are permanently stuck at this stage, endlessly cycling through this elaborate gene rearrangement. And that, he says, makes these cells vulnerable.
“The idea is to trip up this acrobatic process by blocking the enzyme that opens the hairpin,” he says. “If you can block the hairpin from opening, then the ALL cell would suffer a chromosome break. And then it would die.”
Dr. Lieber and his lab at USC previously discovered that Artemis is the enzyme that unlocks that hairpin. After years of research into how Artemis works and how it interacts with other key proteins, his team used high throughput drug screening to identify four compounds that could potentially block the enzyme.
That’s when he called Dr. Kim at CHLA.
“Dr. Kim is a global expert in research using patient-derived leukemia cells—both in culture and in mouse models,” Dr. Lieber notes. “Although a lot of leukemia research is done using commercially available cell lines, it’s far better to test compounds against actual ALL cells from patients.”
Leaving normal cells unscathed
One reason the project holds promise is because of its potential to leave the body’s normal cells untouched.
That’s because those DNA hairpins don’t occur anywhere else in the body except B cells and T cells. Healthy mature lymphocytes no longer have these hairpins, so they would not be impacted. And while blocking the hairpin opening would block production of new, normal lymphocytes, 99% of the body’s B cells and T cells are already beyond this step.
“It’s a beautiful hypothesis,” Dr. Kim says. “It’s so straightforward. And if it works, this approach could potentially be available for all patients, not just a small subset.”
In its new study, the team identified one compound that was most successful at blocking Artemis. The next step is for Dr. Kim and her lab at CHLA to test it against patient-derived ALL cells, but this time in mouse models.
The researchers are also trying to find the ideal agent to inhibit Artemis. These initial compounds, Dr. Lieber notes, are a starting point. The team wants to ensure that the agent inhibiting Artemis doesn’t have toxic effects of its own.
“The goal is to create a therapy that kills the tumor without affecting the normal cells,” Dr. Lieber says. “This is about as specific of a therapy as one can imagine. Although we still need to complete many steps before we could potentially test this in patients, our hope is that this work will lead to a more effective and less-toxic therapy for ALL.”
Study authors were Heather A. Ogana, Samantha Hurwitz, Chih-Lin Hsieh, Huimin Geng, Markus Müschen, Deepa Bhojwani, Mark A. Wolf, James Larocque, Michael R. Lieber and Yong-Mi Kim.