“Meaningful Hope:” Elevidys Gene Therapy Helps Eliot Move With Ease
Elizabeth was stunned.
She’d just watched her 4-year-old son, Eliot, jump fluidly up and down on his pirate ship (read: bed).
This might sound like the typical activity of a preschool-aged kid. For Eliot, though, it was extraordinary.

Throughout his early life, Eliot couldn’t properly jump, despite attempting often. He’d get winded walking down the block and had difficulty climbing stairs. These were the early manifestations of a genetic mutation linked to a severe degenerative neuromuscular disease, Duchenne muscular dystrophy (DMD).
Today, however, Elizabeth saw Eliot’s reality shift:
One month earlier, he had received a groundbreaking gene therapy called Elevidys at Children's Hospital Los Angeles. After watching Eliot jump, she even called the neurologist who developed Elevidys to check if it were possible the therapy was already working. “I was very conscious of the placebo effect,” she says. “But no, I was seeing the therapy’s effect.”
Deep inside Eliot’s body, Elevidys was helping his muscle cells get healthy. Eliot’s body was restoring itself.
Life-improving Elevidys
Today, Eliot is a rambunctious 5-year-old who walks, runs, jumps, and climbs with relative ease—and who actually has to be reminded to slow down.
While Eliot may always have DMD, Elevidys is designed to significantly slow neuromuscular degeneration, and in turn, profoundly improve patients’ quality of life.
Elevidys gene therapy works through a one-time IV infusion. Doctors inject a non-disease-causing virus, called a viral vector, into the patient’s body. That vector’s job is to find the person’s muscle cells. Then, the vector delivers a modified gene that makes a shortened version of dystrophin, a critical protein that patients with DMD have trouble producing.
Early days
Eliot was diagnosed with DMD early and unexpectedly. At 5 months old, he spent five days in the hospital with a bout of RSV. Doctors mentioned noticing abnormal liver function values, which aren’t uncommon for viruses but can also indicate a neuromuscular problem.
When Elizabeth took Eliot to his follow-up appointment at CHLA a week later, his pediatrician, Eyal Ben-Isaac, MD, recommended further monitoring. Dr. Ben-Isaac referred Eliot to Leigh Maria Ramos-Platt, MD, Medical Director of CHLA’s Neuromuscular Disorders Center, for evaluation and genetic testing.
The diagnosis
In January 2020, Dr. Ramos-Platt called Elizabeth to confirm their worst nightmare. Eliot had DMD.
“There are no words to describe that feeling. It’s beyond sadness and grief,” Elizabeth reflects. “I remember saying, ‘I just need to know he’s going to be OK,’ and Dr. Ramos-Platt responding, ‘I think he's going to be OK.’”
The words Dr. Ramos-Platt offered Elizabeth that January day weren’t platitudes. As the two spoke, researchers were trialing game-changing new treatments for DMD. CHLA had already sent children to participate in some of these clinical trials.
“At the time, her words didn't give me immediate comfort,” says Elizabeth, “but it wasn't the conversation I think she would be having with patients 10 years ago.”
“Watching the treatment landscape for DMD shift, I was very optimistic,” says Dr. Ramos-Platt. “It was so different from when I was a medical resident.” Until very recently, people with DMD had few treatment options aside from corticosteroids and physical therapy to reduce inflammation and prolong muscle function. Without more effective treatments, the disease could often lead to loss of motor function and ultimately total immobility, difficulty breathing, and shortened life expectancy.
Elizabeth“I remember saying, ‘I just need to know he’s going to be OK,’ and Dr. Ramos-Platt responding, ‘I think he's going to be OK.’”
The wait
By March 2020, the COVID-19 pandemic had shut down businesses across the globe, slowing progress toward new gene therapies to a crawl. Both Elizabeth and Dr. Ramos-Platt kept close tabs on which trials were progressing, monitoring for any indication from the FDA that a drug would be approved within the next few years.
“We just had to watch and wait. And it was horrible,” Elizabeth says. “Eliot was too young for alternative treatments like steroids. He was so little. We just sat with this terrible information.”
The text
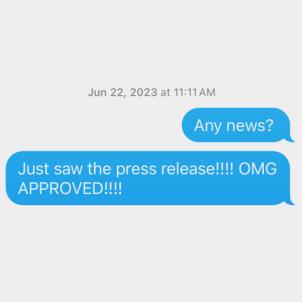
Shortly after Eliot’s fourth birthday, on Jun. 22, 2023, Elizabeth received the text from Dr. Ramos-Platt she’d been dreaming of for three years. Elevidys was officially FDA-approved for 4- and 5-year-olds.
Dr. Ramos-Platt and the Neurological Institute team were ready to spring into action, having conducted several test runs with the drug maker, Sarepta, a month prior. “We’d been building our plan since 2021 based on our experience administering the gene therapy Zolgensma for spinal muscular atrophy,” Dr. Ramos-Platt recalls. “We didn’t have a concrete process yet for Elevidys—and as the first patient to receive the treatment at CHLA, Eliot’s journey would become our blueprint for patient No. 2, No. 3, and so on.”
Dr. Ramos-Platt introduced Elizabeth to treatment coordinators at Sarepta, and soon began an odyssey of approvals, consents, and back-and-forths with insurance. Eliot’s blood also needed to be tested for any viral antibodies that could interfere with the vector’s ability to deliver the gene therapy.
Once it was confirmed that Eliot was free of any conflicting antibodies, Elizabeth says the family lived their lives “in a bubble,” while staying in constant contact with Dr. Ramos-Platt.
Treatment day: “Like watching someone go to the moon”

To an objective observer, Eliot’s treatment might have looked like any number of routine IV infusions. Except, one day earlier, Eliot’s dose of Elevidys arrived at CHLA with its very own security detail, stored inside a massive, armored, refrigerated box set to exactly minus-76 degrees Fahrenheit.
On Aug. 29, 2023, Eliot, Elizabeth, and Eliot’s father Richard arrived at the CHLA Infusion Center as the pharmacy began thawing and preparing the medicine.
“It was the most extraordinary moment. Still, there were so many things about it that seemed ordinary,” Elizabeth reflects. Eliot was treated in a typical infusion room, sitting on a hospital bed with his stuffed animals like any other child. Inside Eliot’s room, Dr. Ramos-Platt introduced them to Susie Tatoy, RN, who has worked in CHLA’s Infusion Center for more than 40 years. Dr. Ramos-Platt says Tatoy is exactly whom you want by your side on a life-changing treatment day.

“I think almost the entire Neurology team showed up,” Elizabeth recalled. “Then I remember Susie saying, ‘All right, it started.’ And it's just like … I don't know … like watching someone go to the moon.”
“It was such an ordinary moment in some ways. And yet what's happening is they're transferring a gene to my son to give him something his body can't produce. And he's one of, I don't know, a hundred or so kids who this happened to in the entire world,” says Elizabeth.
Eliot sat there quietly and patiently for the hour-long process and subsequent hours of observation. “It was mind-blowing,” Elizabeth adds. “It moves me every time I go back there in my memory.”
Elizabeth“It was such an ordinary moment in some ways. And yet what's happening is they're transferring a gene to my son to give him something his body can't produce. And he's one of, I don't know, a hundred or so kids who this happened to in the entire world.
Recovery and milestones
For the next 16 weeks, Eliot and Dr. Ramos-Platt needed to be nearly inseparable. “I become the patient’s best friend,” she often tells families, as patients must undergo weekly tests to ensure their body is responding positively to the therapy. Eliot was a bit shy at first, but eventually, he and Dr. Ramos-Platt bonded over their mutual love of unique animals—especially guinea pigs.

Dr. Ramos-Platt explains that in the first week, patients tend to feel a bit of digestive discomfort, which might persist through the end of the month. She also notes that the care team needs to avoid confusing any early newfound energy with the effects of extra steroids. “But about a month later, they’re doing things that make me say, ‘OK, you didn’t do that before!’”
Before being treated, Eliot scored 22 out of 34 on the North Star Ambulatory Assessment (NSAA), a test used to assess motor function—which indicated that the disease had already begun to impair his movement considerably.
Six weeks after his treatment, he scored a perfect 34 out of 34.
“The physical therapist couldn’t believe her eyes,” Dr. Ramos-Platt recalls. They decided to have Eliot come back another day and conduct the test one more time after he’d stopped taking the extra steroids.
Once again: perfect score.
‘Eliot Day’
Aug. 29, 2024, marked the one-year anniversary of Eliot’s treatment—a day Elizabeth has coined “Eliot Day.”
The family acknowledges Eliot Day not with a cake or a celebration, but with quiet reverence.
“He could tell that things were changing,” says Elizabeth when asked how Eliot processed receiving what she calls “the magic medicine” as a preschooler. “He's so aware of the seriousness of what happened, and I think it's probably very overwhelming for someone so young.”
“Internally, as the parent of a child with something so serious, it's one of those days that is just special,” Elizabeth says. “I think a lot about that day, I talk about it with my husband, and it’s more solemn than celebratory. I don't mean that negatively—it was just such a profound experience. There is no party or celebration that could capture what gene therapy did for my son.”
ElizabethI think a lot about that day, I talk about it with my husband, and it’s more solemn than celebratory. I don't mean that negatively—it was just such a profound experience. There is no party or celebration that could capture what gene therapy did for my son.
Not fantastical hope, but meaningful hope
Elizabeth encourages other families grappling with a DMD diagnosis to acknowledge that today’s treatment for DMD is “a whole new world and trajectory” than even five years ago.
“Someone once said to me, ‘The best treatment is the one in front of you.’ Do I think that there may be better treatments in 10 years? That is a possibility. But right now, we have a treatment that could be life changing for many of these kids."
“It’s important to believe the science—to believe there is not fantastical hope, but meaningful hope. If Eliot is any poster child, gene therapy has been a game changer for him.”