How a New Test Is Transforming Care for Retinoblastoma
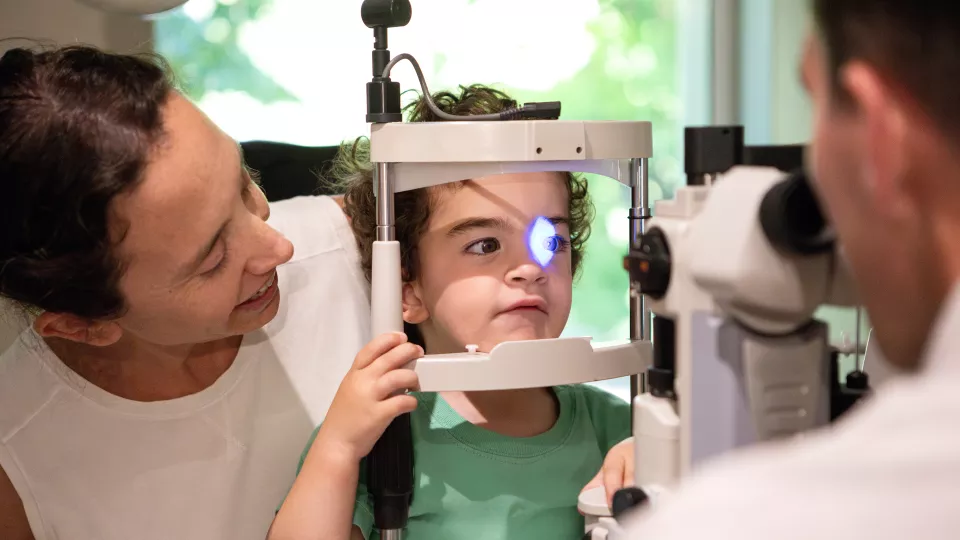
In 2017, a Children’s Hospital Los Angeles team led by Jesse Berry, MD, pioneered the development of the first liquid biopsy for retinoblastoma, the most common childhood eye cancer. But while the biopsy information—taken from the aqueous humor fluid in the front of the eye—could be studied in the lab, it wasn’t yet able to be applied to patient care.
Now, a team in the Center for Personalized Medicine at CHLA has created the world’s first validated clinical test that can be used with this liquid biopsy. Called LBSeq4Kids, the test launched late last year and is now helping to guide treatment for children with this cancer.
Dr. Berry, Director of Ocular Oncology and the Retinoblastoma Program at CHLA, is currently leading a National Institutes of Health-funded international study of this aqueous humor biopsy. She shares how the validated clinical test is benefiting patients today, what it means for the future, and why retinoblastoma treatment is finally “catching up” to other cancers.
Is this platform already changing care for retinoblastoma?

Yes. At CHLA, we’re using it for all our patients. At diagnosis, we take an aqueous humor liquid biopsy, and we run it through this new platform, LBSeq4Kids. This stands for Liquid Biopsy Sequencing—for sequencing of DNA—for Kids. While there are some liquid biopsy platforms for adults with cancer, this technology really lags behind in children, so I love that CHLA literally has “for kids” in the name.
Currently, the key thing we can determine is if a child has one of two biomarkers that indicate their cancer is very aggressive or much more likely to recur. Retinoblastoma is a cancer that tends to recur nearly 50% of the time.
Now, for the first time, we can know right at diagnosis if a child has a specific type of tumor that is at higher risk for recurrence or a poor outcome. We are continuing to evaluate just how impactful these markers are when detected at diagnosis.
What are the two biomarkers you look for?
One is MYCN amplification. This is an oncogene that makes many tumor types more aggressive. In retinoblastoma, we know that tumors with MYCN amplification are more likely to escape the eye and spread elsewhere in the child’s body. But in the past, we didn’t know if the tumor had this marker unless we removed the eye.
Now, using the liquid biopsy and LBSeq4Kids, we have this information at diagnosis, so we can discuss with parents and recommend removing the eye. That can save a child’s life. It can also spare a child a lot of treatment aimed at saving an eye that ultimately would have been removed anyway.
The second marker we look for is called 6p gain, which means there are too many copies of the 6p chromosome. Our lab discovered that when a tumor has this marker, it’s much more likely to recur. If we find 6p gain in the aqueous humor, we can follow that child with exams under anesthesia for much longer. We can then catch any recurrence of the tumor very early, when it’s still tiny and more easily treated.
How are you collaborating internationally in this research?
We now have 30 centers around the world that have committed to being part of our international NIH-funded liquid biopsy study. Each center takes samples and sends them to us to evaluate. Over time, as we gather more data, we’ll be able to prove that when we find X marker in the liquid biopsy, it means Y for the patient. This is really important.
One example of the many collaborations we have formed is with the European Retinoblastoma Group, which includes all centers that treat RB in Europe. We’re working to help them standardize guidelines around this liquid biopsy— how you perform the biopsy, how much fluid to take, how you process it to detect markers, etc.
Over the next year, they will start sharing their data with us as well. So when one of the European centers takes an aqueous humor sample, we will be able to add that data to our large and growing repository of retinoblastoma tumor information.
How will retinoblastoma care look different in the future?
The liquid biopsy and our validated clinical test really open the door to more personalized treatment. For example, I partner closely with oncologist Rachana Shah, MD, at CHLA. She is proposing to study a more intensive treatment protocol for children with the 6p gain biomarker.
The idea is: Could additional treatment upfront help prevent the cancer from recurring in these patients? And alternatively, could children without this marker have less intense therapy? This is done for other pediatric and adult cancers. In breast cancer, for example, the treatments patients receive are based on what’s found in their tumor biopsy. Now there’s an opportunity for retinoblastoma treatment to catch up.
In the future, we’ll increasingly be able to personalize a patient’s retinoblastoma treatment based on these markers we find at diagnosis. And as we understand more about what’s driving these tumors, that will lead to newer and more targeted treatments as well. We have a tremendous opportunity to improve care and outcomes for children with this cancer.