Cellular Imaging Core Equipment and Software
The Cellular Imaging Core houses modern light microscopy and digital image processing equipment to support cell biology research at our facility as well as other institutions.
All microscopes are equipped with high-resolution objective lenses and digital cameras to produce the highest quality images and time-lapse movies.
Leica STELLARIS 8 DIVE intravital two-photon, confocal, FLIM microscope (Saban 448)
Equipped with 4 x HyD S (silicon avalanche photodiode) and 1 x HyD X (GaAsP) confocal detectors plus 4 x HyD X non-descanned detectors (NDDs)
Every detector is capable of superresolution imaging with LIGHTNING adaptive deconvolution and of fluorescence lifetime (FLIM) imaging
Equipped with an incubator for long-term live imaging
Upright configuration for intravital imaging of live mice and of cultured organs and tissue slices
Equipped with a resonant scanner for extremely fast scanning and a motorized stage for tilting/stitching of large volumes
The confocal lasers available are:
- 405 nm for UV-excitable dyes such as DAPI
- 448 nm for CFP
- 488 nm for green dyes such as Alexa Fluor 488, FITC, and GFP
- 514 nm for YFP
- 561 nm for red dyes such as Alexa Fluors in the 500s, Cy3, rhodamine, RFP, tdTomato and mCherry
- 638 nm for far-red dyes such as Alexa Fluor 633 and 647, Cy5 and TO-PRO-3
- 730 nm for infrared (IR) dyes such as Alexa Fluor 750
Pulsed infrared laser for non-linear techniques: two-photon, second harmonic generation (SHG), Fluorescence Lifetime Imaging Microscopy (FLIM):
- Coherent Chameleon Discovery NX Ti:Sapphire laser
- Tunable 660-1320 nm
- Fixed 1040 nm line
The lenses available are (working distance in mm):
- 10×/0.40 HC PL APO CS2 (2.56)*
- 25×/0.95 Water HC FLUOTAR L VISIR (2.5)*
- 40×/1.10 Water CORR HC PL APO CS2 (0.62)*
- 40×/1.25 Glyc motCORR HC PL APO CS2 (0.35)*
- 63×/1.40 Oil HC PL APO CS2 (0.14)*
- 25×/1.00 Water motCORR HC IRAPO L (2.6)
- 16×/0.8 IMM motCORR HC FLUOTAR L VISIR (8.15)
*with fast Piezo focus
Zeiss LSM 780 inverted two-photon confocal microscope (Saban 348)
Incubator for long-term live imaging
Motorized stage for tilting/stitching large volumes
The visible lasers available are:
- 405 nm for UV-excitable dyes such as DAPI
- 458 nm for CFP
- 488 nm for green dyes such as Alexa Fluor 488, FITC, and GFP
- 514 nm for YFP
- 543/561 nm for red dyes such as Alexa Fluors in the 500s, Cy3, rhodamine, RFP, tdTomato and mCherry
- 633 nm for far-red dyes such as Alexa Fluor 633 and 647, Cy5 and TO-PRO-3
Infrared laser for non-linear techniques (two-photon and second harmonic generation, SHG):
- Coherent Chameleon femtosecond Ti:Sa laser, tunable
- Coherent Chameleon Compact Optical Parametric Oscillator (OPO), tunable 1000-1600nm
Leica STELLARIS 5 inverted confocal microscope (Saban 338)
Purchased with NIH S10 funding. Click here to access the study
Equipped with 4 HyD S spectral detectors, each capable of superresolution imaging with LIGHTNING adaptive deconvolution, and a motorized stage for tilting/stitching large volumes.
Objective lenses:
- 10x/0.4 and 20x/0.75 air
- 20x/0.95 water lens for deep imaging (2.4 mm working distance)
- A 63x/1.4 oil lens
Lasers:
- 405 nm for UV-excitable dyes such as DAPI
- 488 nm for green dyes such as Alexa Fluor 488, FITC, and GFP
- 561 nm for red dyes such as Alexa Fluors in the 500s, Cy3, rhodamine, RFP, tdTomato and mCherry
- 633 nm for far-red dyes such as Alexa Fluor 633 and 647, Cy5 and TO-PRO-3

Leica STELLARIS 5 inverted confocal microscope (Saban 341)
The Leica STELLARIS 5 microscope represents the latest in confocal imaging technology, bringing together several cutting edge techniques into one state-of-the-art system:
- Five independent photon-counting detectors (HyD S) for spectral imaging.
- Pulsed white light laser (WLL; 485-685 nm) plus a 405 nm laser for excitation of virtually ANY fluorescent dye up to Alexa Fluor 750.1
- Flourescence lifetime imaging (FLIM) with TauSense to probe cellular function, perform fast & sensitive FRET analysis, and separate even spectrally identical signals.2
- Resonant scanner for extremely fast scanning.
- Superresolution imaging with LIGHTNING adaptive deconvolution.
The STELLARIS is outfitted with:
- 10x/0.4 and 20x/0.75 air lenses.
- 20x/0.75 and 40x/1.1 water lenses.
- A 63x/1.4 oil lens.
- A motorized stage for tilting/stitching large areas.
- An environmental chamber for live imaging (overnight and weekend time courses permitted).
- Focus stabilization for long time courses.
1CFP excitation is achieved with 405 nm (~50% efficiency).
2CFP FLIM is not possible as the 405 nm laser is not pulsed; CFP/YFP FRET is accomplished in a conventional manner.
Zeiss Axio Observer 7 fluorescence live cell imaging microscope (Saban 548)
The Zeiss Axio Observer 7 live cell imaging microscope is capable of imaging live specimens using widefield fluorescence, brightfield, phase-contrast, and/or DIC/Nomarski with an sCMOS camera for speed, sensitivity, and a large field of view at high resolution. It is equipped with a robust focus stabilization system and an environmental chamber for stable long-term imaging. A programmable motorized stage allows for automated imaging of multiple positions in parallel, for example of different treatment groups in a multi-well chamber. The objective lenses on this microscope range from 5x to water-immersion 63x. It utilizes LED illumination for extremely fast switching between color channels. An ApoTome structured illumination system is available for optical sectioning to remove out-of-focus haze. The fluorescence excitation LEDs available are:
- 385 nm for UV-excitable dyes such as DAPI
- 425 nm for CFP
- 469 nm for green dyes such as Alexa Fluor 488, FITC, and GFP
- 511 nm for YFP
- 555 nm for red dyes such as Alexa Fluor 546, Cy3, rhodamine, RFP, and tdTomato
- 590 nm for longer red dyes such as Alexa Fluor 568 and 594 and mCherry
- 631 nm for far-red dyes such as Alexa Fluor 633 and 647, Cy5 and TO-PRO-3

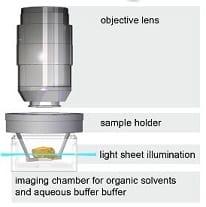
Miltenyi UltraMicroscope II (Saban 538)
Lightsheet microscopy is capable of imaging large specimens, such as whole mouse embryos and organs, in 3D rapidly and at high resolution. In this technique, a thin sheet of light optically slices through a cleared specimen. A 3D image is built by moving the sheet through the depth of the specimen while a synchronized camera captures an image of each optical section. Utilization of sCMOS camera technology, with its high sensitivity and fast data transfer rate, allows for short exposure times and much faster acquisition times than point-scanning confocal. Since illumination is orthogonal to the direction of image capture, each plane is illuminated only once and the specimen suffers substantially less photobleaching. Additionally, the UltraMicroscope II is unique in its ability to image specimens cleared with organic solvents for superior clarity over aqueous techniques. Superb specimen clarity combined with minimal photobleaching and a high numerical aperture long working distance lens allow the UltraMicroscope to generate high-resolution images at increased depth, up to about 6 mm; it can accomodate specimens up to 1.2 x 1.2 x 0.6 cm. It is equipped with a zoom body to easily change between acquiring fast broad-view images and achieving single-cell resolution (down to 0.5 microns/pixel). The fluorescence excitation lasers available are:
- 405 nm for blue dyes such as DAPI and CFP
- 488 nm for green dyes such as Alexa Fluor 488, FITC, GFP, and YFP
- 561 nm for red dyes such as Alexa Fluors 546 and 568, and 594, Cy3, rhodamine, RFP, tdTomato, and mCherry
- 640 nm for far-red dyes such as Alexa Fluors 633 and 647, Cy5 and TO-PRO-3
Combined Fluorescence/Color Transmitted Light Microscopes: inverted Leica DMI6000B (SRT 1030A) and Nikon Eclipse Ti-E (SRT 309C) and upright Leica DM4000B LED (Saban 452)
These two microscopes are highly versatile instruments, each capable of imaging any type of biological specimen using all manner of traditional light microscopy techniques:
- Fluorescence
- Brightfield
- Phase-contrast
- DIC/Nomarski
Each microscope has two cameras:
- Sensitive camera for low-light fluorescence
- Color camera for transmitted light
Each microscope has filter sets for fluorescence imaging of:
- UV-excitable dyes such as DAPI
- Green dyes such as Alexa Fluor 488, FITC, GFP, and YFP
- Red dyes such as Alexa Fluor 546, 561 and 594; Cy3; rhodamine; RFP; tdTomato; mCherry
- Far-red dyes such as Alexa Fluor 633 and 647, Cy5 and TO-PRO-3
Other features:
- Inverted configuration for visualizing specimens mounted on slides or in culture dishes.
- Motorized stage for automated tiled imaging of large areas at high resolution.
- Objective lenses ranging from 2.5x to oil-immersion 100x
Olympus IX73 inverted fluorescence microscope (Saban 542)
This microscope has an inverted configuration and long working distance lenses of 5x, 10x, 20x, and 40x for visualizing specimens mounted on slides or in culture dishes. The camera is fast and sensitive for quick snapshots of fluorescent and brightfield specimens. The fluorescence filters are for:
- UV-excitable dyes such as DAPI
- Green dyes such as Alexa Fluor 488, FITC, GFP, and YFP
- Red dyes such as Alexa Fluor 546, 561 and 594; Cy3; rhodamine; RFP; tdTomato; mCherry
- Far-red dyes such as Alexa Fluor 633 and 647, Cy5 and TO-PRO-3

Leica MZFLIII Fluorescence Stereoscope & Optical Projection Tomography (OPT) Microscope (Saban 439)
- Specializes in 3D imaging of organs, embryos and other whole specimens
- Filters for UV, green, red, and far red fluorescent dyes and protein
- Capable of reflected and transmitted light
- Motorized focus (z) drive for “extended depth of focus” images
- Capable of tomographic 3D imaging (OPT) of fluorescent and colorimetric labeling


Zeiss Axioplan Upright Microscope (Saban 5th Floor Desk C)
- Specializes in color brightfield imaging
- Color CCD camera
CARV II Spinning Disk Live Cell Imaging Confocal (Smith Tower 1030B)
- Nipkow spinning disk confocal system with lamp-based excitation (no lasers)
- Enclosed in environmental chamber to control temperature, CO2, and O2 for imaging live cells over extended time
- Capable of standard blue, green, red and far red fluorescence in confocal or widefield mode
- Optics for DIC/Nomarski and phase contrast imaging
Image Processing Workstations and Software (Saban 543 and Smith Tower 322C)
- Arivis Vision4D - for visualization and analysis of static and timelapse 3D images, including cell/particle tracking. Specialized for efficiently processing very large datasets, such as lightsheet images.
- MetaMorph – powerful 2D and 3D image processing and analysis including morphometry and movie making
- AutoDeblur – very user-friendly automated deconvolution of widefield, confocal, and lightsheet data to reduce blurring and noise and increase resolution
- Photoshop – image preparation for publication and presentation
- FoveaPro – scientific image processing and analysis add-ons for Photoshop
- ImageJ – versatile, customizable, open-source scientific image processing and analysis software. The core’s Imaging Scientist can write custom software for ImageJ to suit your needs