Award Acceptance and Progress Reports
Upon notification to the Grants team and Principal Investigator that a proposal is awarded for funding, a member of the Grants team will process the acceptance of the award and work with the investigator on next steps. Following the start of the project, the Grants team will also support investigators with Progress Reports and any changes to the project scope, details of which are below.

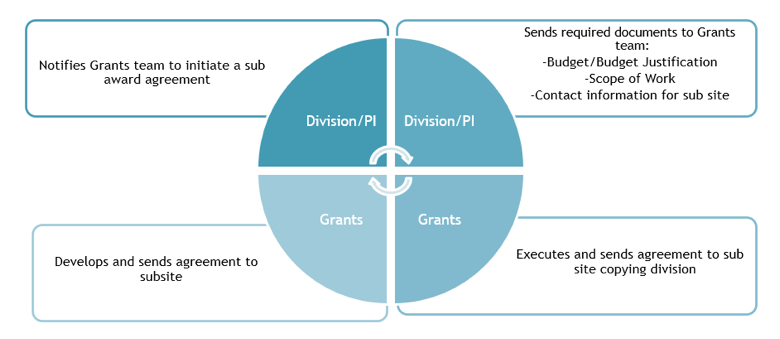
Subaward Processing
Progress Reports
As a component of the reporting process to the sponsoring agency, progress reports are typically required for all awards. The reporting requirements for an agency are generally listed in the program announcement, the agency’s polices and guidelines, notice of grant award, or sponsored research agreement. The Grants team must review and approve all progress reports prior to submitting to the agency.
NIH Awards
Progress reports usually are required annually as part of the non-competing continuation award process. NIH may require these reports more frequently. Refer to the notice of award (NOGA or NOA) for reporting requirements. The “Non-Competing Continuation Progress Report” (PHS 2590) or equivalent documentation (e.g., Research Performance Progress Report [RPPR]) must be submitted to, and approved by, NIH to non-competitively fund each additional budget period within a previously approved project period (competitive segment). Except for awards subject to Streamlined Non-Competing Award Process (SNAP), the progress report includes an updated budget in addition to other required information.
The RPPR is a federal wide format for the submission of required annual or other interim performance reporting on grant and cooperative agreement awards. NIH now requires use of the RPPR module in eRA Commons to submit all annual progress reports. NIH continues development of the RPPR for final progress reports and for administrative extensions (Type 4s; e.g., SBIR/STTR Fast-Track Phase II application) and will update the community as progress is made.
NIH Progress Report Deadline
Streamlined Non-Competing Award Process (SNAP) RPPRs are due approximately 45 days before the next grant year budget period start date. Non-SNAP RPPRs are due approximately 60 days before the next grant year budget period start date. Multi-year funded (MYF) RPPRs are due annually on or before the anniversary of the budget/project period start date of the award. The exact start date for a specific award may be found in grants status in eRA Commons.
A report of all progress reports due for an organization is available at here (CHLA’s IPF Number is 1520001).
NIH RPPR Progress Reports
Route the completed RPPR to the Grants analyst by selecting an Authorized Signing Official. Use the validation button in the RPPR application to ensure there are no errors in the application prior to submitting it to Grants.
Change in Scope
Almost without exception, a change in scope of work requires a prior approval from the sponsoring agency.
In general, the Principal Director (PD)/Principal Investigator (PI) may make changes in the methodology, approach, or other aspects of the project objectives. However, a change in scope almost always requires sponsor approval unless special permission is given within the award agreement. A change in scope is a change in the direction, aims, objectives, purposes, or type of research training, identified in the approved project.
Actions likely to be considered a change in scope or objectives include, but are not limited to, the following:
- Change in the specific aims of the project and approved at the time of award
- Substitution of one animal model for another
- Any change from the approved use of animals or human subjects
- Shifting the emphasis of the research from one disease area to another
- Applying a new technology, or changing assays from those approved to use of a different type of assay
- Transferring the performance of substantive programmatic work to a third party by contract or any other means
- Change in key personnel whose expertise is critical to the approved project
- Significant re-budgeting whether or not it requires approval under rules governing budget changes. Significant re-budgeting occurs when the cumulative amount of transfers among direct cost categories for the current budget period exceeds either 25 percent of the total amount awarded or $250,000, whichever is less
- Incurrence of patient care costs not previously approved and/or when a grantee desires to re-budget funds out of the patient care category
- All requests for prior approval must be in writing, well-justified, and accompanied by supporting documentation to ensure that changes are reasonable and appropriate. The written requests have to be submitted to Grants to be reviewed and submitted.
To obtain sponsor approval, the PI must draft a justification letter on institutional letterhead. The letter must be signed by the division head and forwarded to the assigned Grants analyst.
Change in Personnel
Occasionally, it is necessary to change the Principal Investigator (PI)/Project Director (PD) or key personnel of an awarded grant or contract. This may be the result of a PI withdrawing completely from a project or taking a leave or sabbatical for a continuous period of three (3) months or more.
Most federal sponsors require the grantee to notify them prior to any changes with the PI or other key personnel named in the notice of award, as well as approval of any alternate arrangement to the PI or other key personnel proposed by the grantee before such changes can be implemented. Divisions should refer to the terms and conditions of the award to determine if the sponsor will allow the award to be retained in the absence of the original PI. Generally, sponsors reserve the right to terminate a grant if approval for a leave of absence has not been sought or if the replacement PD/PI or key personnel is not acceptable.
When the sponsor requires prior approval, such requests should be sent in writing to the Grants team for review and approval before they are submitted to the sponsor. Contact your Grants analyst for assistance.
Change in Principal Investigator
To request a change in PI/PD, the current PI or division must initiate the request and route it to the Grants team.
A request to a sponsoring agency for designation of a new PI/PD will typically state the reason(s) for such change. The letter should also include a clear outline of the new PI's qualifications to lead the program and accomplish the objectives as outlined in the original proposal. For NIH grants, a strong scientific justification related to the scientific project, including any proposed changes in scope and any budget changes resulting from the proposed change should be included in the letter.
Additionally, these items may be required for submission to the sponsor in support of the proposed PI:
- Curriculum vitae (CV)
- Current and pending support
- Letter signed by the department chair justifying the change. Also include a signature block for a CHLA authorized signing official. Submit this letter, plus a Research Conflict of Interest Certification form for the new PI, to the Grants team. Upon receipt of the letter, Grants will submit the request to the sponsor in accordance with their guidelines. Upon sponsor approval for change of PI, Grants will notify all of the appropriate business officials of the change and make necessary updates to all data systems and files.