Proposal Preparation and Submission
In order to best support the research community at CHLA, please find below helpful resources, definitions and descriptions to aid in the proposal process. Please note that Research Operations, as the office of CHLA’s institutional signing official, has the responsibility and oversight to review all grant applications to ensure financial and administrative portions of all proposals meet hospital and sponsoring agency guidelines. Per CHLA policy, grant applications must be received by the Grants team at least five (5) working days prior to the funder’s deadline. Principal investigators should confer with their divisional administration team far in advance to ensure proposals are in optimum form before submitting to the Grants team in Research Operations.
Budget Development
Funding from awards must be expended in accordance with governing regulations and agency policy. Pre-award reviews budgets to determine costs are allowable, allocable, and appropriate to grants in accordance with the Office of Management and Budget (OMB) Circular A-81, the agency’s policies and guidelines, and institutional policy.
A cost is allowable when:
- It serves a CHLA business purpose, including instruction, research, and public service
- It is permissible according to CHLA policy and federal regulations (regardless of whether it is an extramurally sponsored project)
- It is permissible (for a sponsored project) according to the terms and conditions of the agreement
- It complies with federal rules for allowability which dictate that costs must be reasonable. A cost is reasonable when: a) prudent person would purchase the item at that price; b) the cost is necessary for the performance of the activity; and c) incurrence of the cost is consistent with established policies and practices
Costs must be allocable to the sponsored agreements. A cost is allocable when:
- It is incurred solely to advance the work under the sponsored agreement
- It benefits both the sponsored agreement and other work of the institution, in proportions that can be approximated through use of reasonable methods
- It is necessary to the overall operation of the institution and in accordance with OMB Circular A-81, is deemed to be assignable in part to sponsored projects
Costs must be given consistent treatment through application of those generally accepted accounting principles (GAAP) appropriate to the circumstances.
All costs incurred on a grant must conform to any limitations or exclusions in 2 CFR 200 or the sponsored agreement.
PIs should work with their division administrators to develop a budget which ensures all costs in the budget are fair and reasonable for a research project in accordance with federal regulations, as applicable, agency policies and guidelines and institutional policy.
Preparing Budgets
When preparing a budget for a grant application, consider the following:
- Build the budget following sponsor guidelines. The typical order of precedence is: 1) funding opportunity announcement; 2) agency’s general grant guide; 3) agency’s policies and guidelines; 4) 2 CFR 200; 5) institutional policies
- Know budgetary limits for the project period, spending caps, etc. (i.e., travel, personnel)
- Include only costs that are allowable, reasonable, and allocable to the project. Check the agency’s policies and guidelines; these will often list allowable and unallowable costs. For NIH projects, refer to the NIH Grants Policy Statement § 7.9 Allowability of Costs/Activities for detailed information about allowable and unallowable costs
- Provide a detailed justification for each category of cost. Requesting items that are typically not allowed (i.e., food, alcohol) requires an extremely detailed description justifying why the cost is necessary
Select appropriate budget template from the Forms & Policies page under the Grants section.
Indirect Costs
Indirect Costs Policy FIN – 048.0 Breakdown | |
Federal Rate | |
RATE | DESCRIPTION |
71% | Organized research |
40% | Other sponsored activities (includes service) |
8% | Training |
35% | Off-campus (rent direct charged to grant) |
Non-Federal Rate | |
RATE | DESCRIPTION |
71% | Industry pre-clinical/lab research |
35% | All other non-federal awards |
Varies | The request for proposal or funding opportunity announcement details a different indirect cost rate. |
Varies | The not-for-profit funding agency has a written policy that it pays a different fixed indirect cost rate. |
Cost Sharing
CHLA seeks to support its faculty in the pursuit of their research interests while, at the same time, allocating all available resources to comprehensive support of the whole enterprise. Cost sharing, as defined below, may be requested by the grantor in the form of funds or other hospital resources that would be contributed or allocated to a sponsored project over and above the support provided by the grantor. This commitment made by CHLA creates the requirement to track cost sharing in accordance with FIN – 043.0 – Cost Sharing/Cost Matching.
CHLA actively discourages voluntary cost sharing on the part of principal investigators; exceptions may occasionally be made if the project in question is deemed by the Vice President, Research Operations to warrant a voluntary commitment. All exceptions must be approved prior to submission of the grant proposal by the Vice President, Research Operations. Reach out to TSRpreaward@chla.usc.edu to get an IDC/Cost Share form.
NIH Federal Budgets
This section refers to preparing a budget for the National Institutes of Health (NIH). Additional resources are located on the NIH website.
Direct Cost Categories
Personnel
Personnel costs often comprise 65-80% of total funds requested. All personnel with effort on project must be listed by name, and all personnel paid on a project must be CHLA faculty.
Principal Investigator(s)
- Effort should reflect work required for project
- If applicable, observe any salary cap limitations (e.g., the NIH Salary Cap)
- Fringe benefits must correspond to approved institutional fringe benefit rate tiers
- May add/delete personnel in future project years if scientifically necessary
- May increase/decrease effort if scientifically necessary in future project years
- Salary inflation should not exceed the sponsoring agency’s inflationary limit
Consultants
- May not be employees or faculty of the hospital
- If consulting costs are substantial (>$10,000), a subcontract may be required
- If awarded, division will handle consulting arrangement and payment through Purchasing
Equipment
- Ask for what is reasonably needed for the project, especially if setting up a new lab
- Itemize each piece with a cost over institutional threshold for equipment ($5,000). The threshold of $5,000 is for unit items, not individual items that add up to $5,000
- Equipment is usually easier to justify in the early research years rather than later years
- Equipment should be required 100% for project
- Equipment is excluded from the F&A base
Materials & Supplies
- Budgeted supplies must be essential to the proposal
- Supplies should be itemized by category (glassware/plasticware, serum/radioisotopes, chemicals, sequencing kits)
- Animal purchase is usually in the supplies category
Travel (Domestic & Foreign)
- Include in the narrative the purpose of the travel and how it is directly related to the proposed project
- The standard request is one domestic trip for PI
- Additional travel can be requested as required by the individual project
- Purpose of travel must directly benefit the project
- Foreign travel must be necessary and extremely well-justified
Patient Care Costs (Inpatient & Outpatient)
- Represents payments to hospitals and/or clinics for either in-patient or outpatient care
- Patient care cannot include travel, lodging, or donor/volunteer/incentive fees. These costs should be in the Other Expenses category
- Additional information on patient care costs is located on the NIH website
Alternations and Renovations
- A&R costs are very rare
- Equipment installation is not normally considered renovations
- Alterations and renovations greater than $25,000 must be approved by NIH
Other Expenses
Examples of other expense items are:
- Animal care costs
- Publication costs, page charges, color plates
- Equipment maintenance contracts (costs must be based on project usage percent)
- Donor/volunteer fees, including travel, lodging, per diem stipends
- Fee charges for services (mass spec., EM, flow cytometry)
- Patient participant incentives and travel costs
- All other costs identified as a direct charge necessary to do the research
- Tuition, which is excluded from indirect costs
Subcontracts (Consortium/Contractual Costs)
- The F&A costs for the first $25,000 of each consortium may be included in the modified total direct cost base
- Most subcontracts are to other non-profits, but in special circumstances CHLA will subcontract to commercial or foreign institutions. See the vendor vs. subcontractor grid for additional guidance
- Subcontract direct and indirect costs are included in CHLA's direct costs
- Modified total direct cost excludes items of equipment and other capital expenditures, hospitalization and other fees related to patient care, fellowships, and that portion over $25,000 of each subaward or subcontract
Indirect Cost (Facilities and Administrative) Category
Refer to the Funding Opportunity Announcement (FOA) to determine whether indirect costs (IDC or F&A) are allowable. The FOA will also delineate whether there is a cap on the indirect cost rate. If there are no mandatory restrictions to IDC, use the applicable rate per CHLA’s IDC Policy. Any voluntary exceptions to the IDC rate will require an IDC wavier, which should detail the amount of indirect to be waived, and must be approved at proposal time by the division head, division administrator, and Research Operations.
For a reference guide on indirect cost definitions and examples, please refer to this link on the NIH website.
Indirect cost (IDC) waiver may be requested by an investigator or funding agency for a specific project. Please read FIN – 048.0 - Indirect Costs, as applicable, prior to requesting and IDC waiver. IDC waivers must be submitted to the Grants team 10 business days prior to the agency deadline. When requesting and IDC waiver please be sure to complete the following:
- Cost Share/IDC Waiver Form
- Indirect Cost Waiver Calculator
- Detailed budget without IDC waiver
- Detailed budget with IDC waiver
Unallowable Costs
Certain cost items are not allowed to be charged to federally sponsored projects. Also, items factored into the indirect costs that may not be included in the direct costs of a project. To determine whether a cost item is allowable, refer to federal regulations, the agency’s polices and guidelines or program announcement, and CHLA policy. NIH Grants Policy Statement Section 7.9 details the allowability of costs on NIH grants.
Subawards and Proposals

Internal Deadlines
All proposals must be submitted to Research Operations at least five (5) working days prior to submission for review and approval. Proposal packages including a completed Extramural Project Intake Form, the application or RFP guidelines/instructions and any other applicable approvals (indirect cost waivers, cost share requests) should be sent to Research Operations at least five days before agency deadline to allow for thorough and appropriate review. Additionally, since cost sharing of indirect cost waiver requests impact budget preparation, these documents should be submitted to Research Operations 10 days prior to an agency deadline.
Proposal Components | Grant and subaward activity |
IDC waiver or cost sharing requests | Ten (10) days prior to the sponsor’s published deadline |
Final proposal package, including draft of the sciences portion | Five (5) working days prior to the sponsor’s published deadline |
Final text for the science portion | Noon the day before the sponsor’s published deadline |
Proposals submitted less than five (5) working days prior to the sponsor deadline will be reviewed in the order in which they were received.
Statement of Qualifications
For support in completing a Statement of Qualifications, please refer to:
The Saban Research Institute – Institutional Information
Foundation Grant Submission Workflow
While the Grants team within Research Operations, as the office of CHLA’s institutional signing official, has the responsibility and oversight to review all applications to ensure the financial and administrative portions of any proposal meet hospital and agency guidelines, the Office of Foundation Relations (OFR) within the Foundation at Children’s Hospital Los Angeles can work with study teams to help develop strong, competitive applications and shepherd an application to certain philanthropic funding organizations. The ORF has developed strong relationships with program staff within a select list of professionally managed philanthropic organizations and is a great resource for investigators seeking foundation grants. The Grants team can connect interested investigators with the OFR upon request.
To understand the workflow of Foundation Grant Submissions when collaborating with the Office of Foundation Relations, see below.
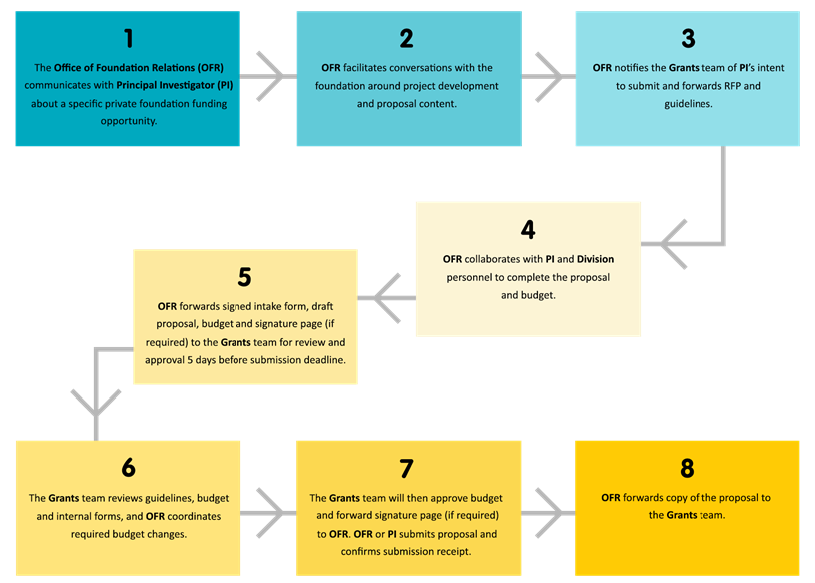
Transferring Awards
Incoming Award Transfers
Transferring a research award from another institution to Children’s Hospital Los Angeles (CHLA) requires coordination between the incoming Principal Investigator (PI), the former institution, the funding agency and CHLA. The PI’s former institution must relinquish the grant back to the sponsor for approval before the sponsor can approve the transfer of the award to CHLA. In many cases, sponsors will allow awards to be transferred when a PI leaves their institution. The Grants team should be notified as soon as PI commits to moving to CHLA and the transfer process should begin as soon as the incoming PI knows the award will be transferring.
The incoming PI’s division must submit a completed Incoming Grant Transfer Checklist Form to the Grants team for each award being transferred.
NIH Transfer Applications
The NIH Change of Grantee Institution Policy requires that a transfer application be submitted through the new institution. This application will receive administrative review to determine if the transfer is appropriate. The NIH’s decision to authorize transfer of the grant will be based upon whether:
- The project has been relinquished by the former institution
- The facilities and resources at the new location allow for the successful performance of the project
- The PI plans no significant change in research objectives and level of expenditures from those described in the previously approved project.
After-the-Fact Proposals
All proposal submissions seeking external support for research and other sponsored projects must be submitted to the Grants team for review and approval prior to submission.
In some cases a sponsoring agency does not require a formal proposal prior to issuing an award. However, even when institutional sign-off is not required by the sponsor, the proposal must still be routed to the Grants for review and approval. Exceptions to this policy may include intramural awards.
Submitting a proposal application late or after-the-fact (ATF) will not circumvent a full review by the Grants team. Proposals submitted without Grants team approval may be administratively withdrawn if the submission is found to be non-compliant with CHLA policy or agency requirements. CHLA always reserves the right to request modification rejection of an award received when there is no approved proposal on file with Research Operations.
ATF Proposal Procedure
- Notify your assigned grants analyst as soon as possible
- Submit entire proposal documents to the analyst along with a completed Internal Intake Form
- If the project has been funded, submit a copy of the proposal documents, along with the award letter, any sponsored-related forms, and all correspondence
- If there are subrecipients on the project, also submit a copy of the subrecipient’s statement of work and budget, and a letter of intent/Subrecipient Form on institutional letterhead confirming their participation in the project
Post Submission Material

Just-in-Time Submissions

The Just-In-Time (JIT) feature of the eRA Commons is available for applications that meet established criteria and fall within a certain percentile or priority scoring range. The JIT feature allows an authorized signing official (ASO) to electronically submit additional grant application information when requested by the grantor agency. The additional information is requested after a peer review of a grant application has been completed and prior to funding. Requests may come in the form of eRA-system generated e-mails or contact made directly from the awarding agency via e-mail and/or phone.
The PI/division is responsible for the initiation of the JIT submission by uploading the requested documents to the Commons and notifying the Grants team via email that the documents are ready for review and submission. JIT information should only be submitted when formally requested by NIH. Documents uploaded into the Commons without proper notification will not be processed.
eRA Commons Upload
To upload JIT documents into the Commons, follow the following steps:
- Login to eRA Commons
- Select Status in the menu bar at the top of the page
- Select the JIT hyperlink on the right side of the screen corresponding to the application for which information is being submitted
- Upload Other Support as requested (file must be in PDF format). There is no form page for providing other support, although sample format pages are available at NIH Sample Other Support Form 1 and NIH Sample Other Support Form 2
Note that effort devoted to projects must be measured in person-months
If human or animals are being used in the project:
- Enter the most recent IRB or IACUC approval date in the text box provided. All approvals must be current. If there is more than one IRB or IACUC approval, use the Other Upload function to include a PDF file with additional the information
- For projects involving human subjects, enter completed training information for all key personnel, including subcontractors, involved with the human subject’s portion of the project
- In order for Pre-award to validate dates, the IRB/IACUC actual approvals must be attached to the e-mail to Grants
When uploads are complete, click Save at the bottom of the screen and select View Just-In-Time Report. Verify and print the report to keep with departmental records.
Once upload is complete contact the Grants team analyst. After proper review, the analyst will submit the information electronically.
Once submitted, the Commons will send an e-mail confirmation to the PI and Grants analyst.
Email Notification
In some instances, it is not possible to submit JIT materials using the eRA Commons, or the PI will receive specific instructions indicating to submit the JIT materials via email.
To submit JIT materials not being submitted using the eRA Commons, send an e-mail to Pre Award with the following information in the subject line: JIT, application number, PI name, and agency due date.
Attach one PDF file with documents arranged in the order expressed by the NIH official: Human Subjects (IRB) or Animal (IACUC) approval(s), whichever is applicable, training certifications, and/or any other supporting documentation should be included.
Also include in the body of the email to Grants any additional narratives providing status, justification or explanation requested by the sponsor.
If submitting a cover letter signed by the PI, forward the PDF and the initial agency request that contains instructions and due date for submission.